Draw A Sketch Of Bohr's Model Of An Atom
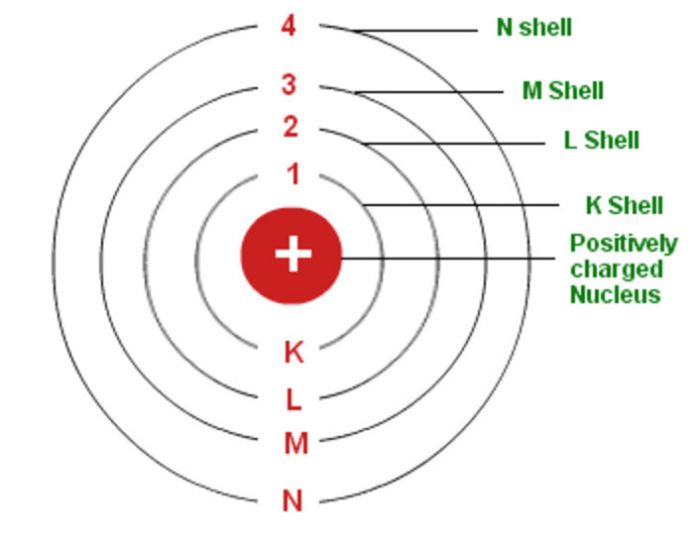
Have you ever wondered how atoms are structured? Well, Bohr’s model of an atom explains this phenomenon. In this model, electrons move in orbits around the nucleus of an atom, which consists of positively charged protons and uncharged neutrons. Here’s a sketch that illustrates Bohr’s model of an atom with three shells.

The Bohr’s model was proposed by Niels Bohr, a theoretical physicist, in 1913. This model provided a new and accurate understanding of how electrons orbit around the nucleus of an atom. In this article, we’ll delve deeper into Bohr’s model of an atom and its significance in modern physics.
The Bohr’s model of an atom consists of a positively charged nucleus made up of protons and neutrons. Electrons move around this nucleus in circular paths, similar to the way that planets orbit around the sun in our solar system.
However, there are some fundamental differences between the Bohr’s model and our solar system. In the solar system, planets can move in any orbit around the sun, whereas electrons’ movements are restricted to certain energy levels or shells in Bohr’s model. The energy level of the electrons increases as we move from the innermost shell to the outermost shell.
These energy levels correspond to discrete levels of energy that the electrons can possess. Electrons emit energy when they move from a higher energy level to a lower one. This energy is released in the form of light or electromagnetic radiation and can be observed in the form of a spectrum.
Bohr’s model is based on quantum mechanics and is considered a milestone in the field of atomic physics. It provided a new way of understanding the structure of an atom and laid the groundwork for further research in the field.
Let’s take a closer look at the different shells in an atom. The innermost shell, also called the K shell, can hold a maximum of two electrons. The second shell, or the L shell, can hold up to eight electrons. The third shell, or the M shell, can hold up to 18 electrons.
The outermost shell, known as the valence shell, is the most significant as it governs the chemical properties of an atom. Electrons in the valence shell determine how an atom will bond with other atoms, and hence, its chemical properties. Some elements have a complete valence shell and are considered stable, while others seek to complete their valence shell by forming chemical bonds with other atoms.
For example, helium, the second element in the periodic table, has a complete valence shell with two electrons. This makes it highly stable and unreactive. On the other hand, elements such as hydrogen, which has one electron in its outer shell, seek to complete their valence shell by forming a bond with another hydrogen atom to form a molecule of hydrogen gas.
Now that you have a better understanding of the Bohr’s model of an atom let’s look at some of the practical applications of this model.
The Bohr’s model of an atom has significant implications in the field of atomic physics. It is the basis of our understanding of the electronic structure of atoms, which in turn is essential for numerous fields, including chemical analysis, materials science, and semiconductors.
The use of semiconductors has become ubiquitous in the modern world, from computers to smartphones to solar energy panels. These devices rely on the electronic properties of semiconductors, which are due to the electronic structure of atoms, as described in the Bohr’s model.
The Bohr’s model of an atom also explains the phenomenon of spectral lines. Spectral lines are the different colors that light splits into when it passes through a prism. Each color is associated with a particular wavelength, which corresponds to a specific energy level of electrons in an atom.
Now that you understand the importance of Bohr’s model of an atom let’s discuss how to draw a sketch of this model.
How to Draw a Sketch of Bohr’s Model of an Atom
Here are some simple steps to follow when drawing a sketch of Bohr’s model of an atom:
- Draw a circle to represent the nucleus of the atom, which consists of positively charged protons and uncharged neutrons.
- Draw three concentric circles around the nucleus to represent the three shells in the Bohr’s model.
- Add electrons in each shell, starting with the innermost shell (K shell). Remember that the K shell can hold a maximum of two electrons, the L shell can hold up to eight electrons, and the M shell can hold up to 18 electrons.
- Label each shell (K, L, M) and the number of electrons in each shell.
- If desired, you can add arrows to indicate the movement of electrons between the shells.
With the steps outlined above, you can easily draw a sketch of Bohr’s model of an atom. However, there are some tips that you should keep in mind to make your sketch look more accurate and aesthetically pleasing.
Tips for Drawing a Sketch of Bohr’s Model of an Atom
Here are some tips that you can use when drawing a sketch of Bohr’s model of an atom:
- Use a compass to draw the circles to ensure that they are perfectly round.
- Use a ruler to draw the circles concentrically to ensure that they are evenly spaced.
- Label the shells clearly and place them in the correct order (K, L, M) with the number of electrons in each shell.
- Add coloring to make your sketch more visually appealing and help differentiate between the shells.
- Try to replicate the actual movements of electrons while drawing arrows to indicate electron movement between different shells.
Conclusion:
Bohr’s model of an atom is a significant contribution to the field of atomic physics. It provided a new and accurate understanding of the electronic structure of atoms, which has numerous practical applications in our modern world. By following the steps outlined in this article, you can draw a sketch of Bohr’s model of an atom and have a better understanding of the inner workings of atoms.


Post a Comment for "Draw A Sketch Of Bohr's Model Of An Atom"